







About the Trial
The TARVA trial is a clinical trial for patients with ankle arthritis that are considering surgery. TARVA stands for Total Ankle Replacement Versus Arthrodesis.
About the Trial
The TARVA trial is a clinical trial for patients with ankle arthritis that are considering surgery. TARVA stands for Total Ankle Replacement Versus Arthrodesis.
Introduction
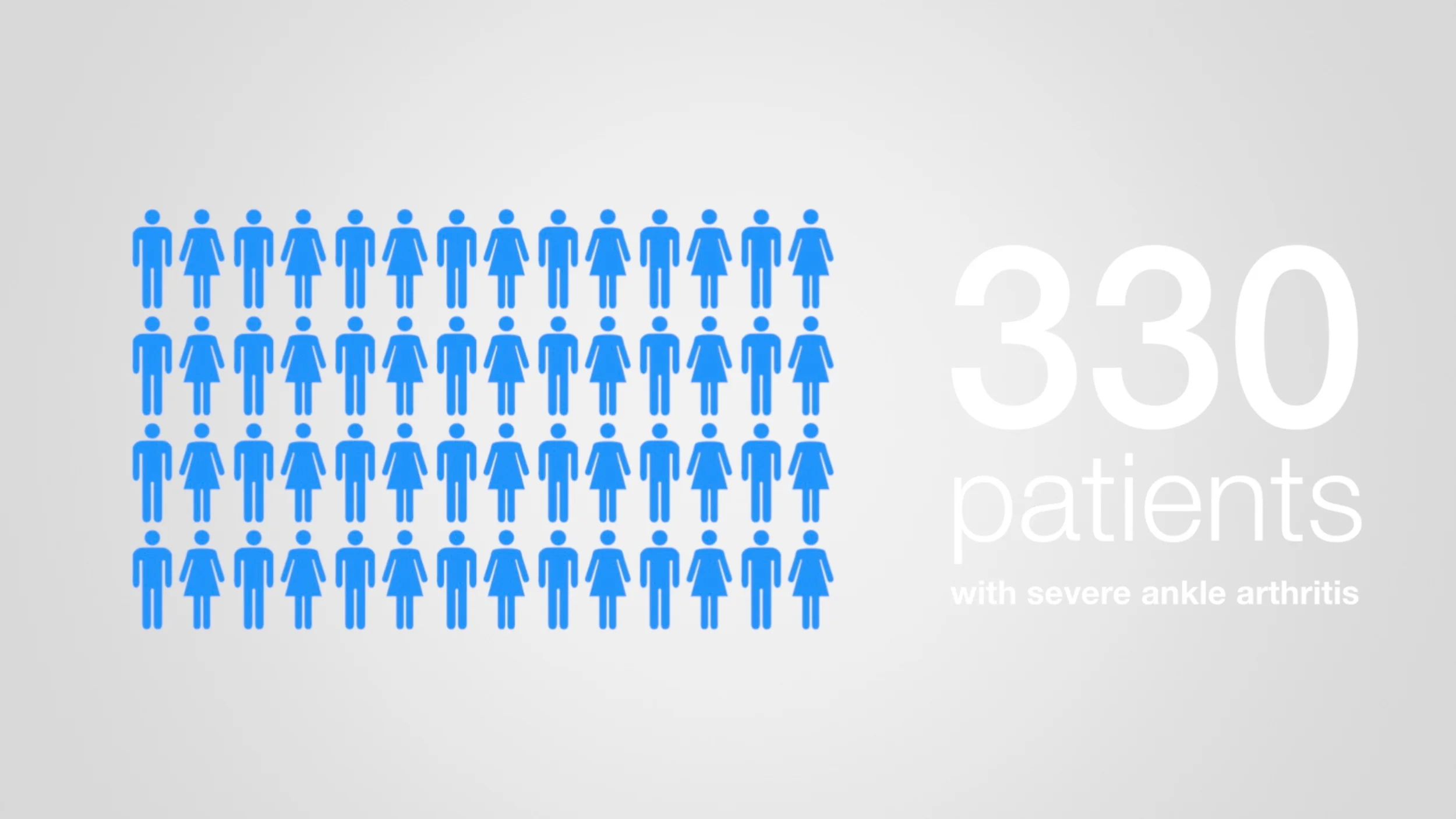
The main surgical treatments for end-stage ankle arthritis are ankle arthrodesis or total ankle replacement (TAR). Approximately 3,000 patients with ankle arthritis undergo ankle arthrodesis or TAR in the NHS each year. Both treatments are available on the NHS, and both have been reported to have good results. Some studies suggest that TAR restores a more natural walking pattern than ankle fusion but other studies have suggested that the need for further surgery is higher with TAR than with fusion. Information on all TARs implanted in the NHS has been captured on the National Joint Registry since 2010, but no similar national registry exists for ankle fusion. There has never been a published randomized trial directly comparing the two procedures.

Ankle Arthritis
Most ankle arthritis is as a result of what is known as "wear and tear" (or osteoarthritis). Other forms of ankle arthritis exist which can effect the ankle such as inflammatory arthritis, an example of which would be rheumatoid arthritis
Ankle Arthritis
Most ankle arthritis is as a result of what is known as "wear and tear" (or osteoarthritis). Other forms of ankle arthritis exist which can effect the ankle such as inflammatory arthritis, an example of which would be rheumatoid arthritis

Treatments
We will discuss the benefits and risks of both procedures and whether any additional procedures will be needed at the time of the operation.
Treatments
We will discuss the benefits and risks of both procedures and whether any additional procedures will be needed at the time of the operation.
Ankle Replacement
Total ankle replacement is an operation to replace a worn-out ankle joint by resurfacing the ends of your tibia and…
Vs.
Ankle Fusion
Ankle Fusion (Arthrodesis) Ankle fusion is an operation to convert a stiff painful joint into an even stiffer but painless…

Trial Oversight
Ensuring Compliance, Patient Safety and Data Integrity.
Trial Oversight
Ensuring Compliance, Patient Safety and Data Integrity.
Trial Steering Committee
Is responsible for oversight of the trial in order to safeguard the interests of trial patients. The TSC is comprised of a 75% majority of independent and lay members, as well as the Chief Investigator, representatives of Principal Investigators, a representative of the sponsor, the health economist and senior trial statistician. The committee provides advice to the Chief Investigator, UCL Comprehensive Clinical Trials Unit, the funder and sponsor on all aspects of the trial through its Independent Chair. The remit of the TSC is to oversee study conduct and advise the TMG.
The Trial Management Group
Is responsible for the day-to-day management of the trial, including monitoring of all aspects of the conduct and progress of the trial, ensuring that the protocol is adhered to and taking appropriate action to safeguard participants and the quality of the trial itself.
Independent Data Monitoring Committee
Is the only oversight body that has access to un-blinded accumulating comparative data. The IDMC is responsible for safeguarding the interests of trial patients, monitoring the accumulating data and making recommendations to the TSC on whether the trial should continue as planned.

Specialists
The TARVA study group includes many of the most experienced foot & ankle orthopaedic specialists in the UK. Each of the individuals listed below is a member of the TARVA publication group, and have contributed towards the TARVA protocol and research publications
Specialists
The TARVA study group includes many of the most experienced foot & ankle orthopaedic specialists in the UK. Each of the individuals listed below is a member of the TARVA publication group, and have contributed towards the TARVA protocol and research publications

Centres
The TARVA study is being carried out in 17 NHS hospitals across the UK.


Ankle Replacement Downloads
-
-
Date: 2022
Sarveen Gajebasia, Toby Jennison, James Blackstone, Razi Zaidi, Patrick Muller, Andrew Goldberg
-
-
-
Razi Zaidi, Rikin Hargunani, Michele Calleja, Jonathan Foley, Andy Goldberg
-
Ali-Asgar Najefi, FRCS(Tr&Orth), Yaser Ghani, FRCS(Tr&Orth), and Andrew J. Goldberg, MD FRCS(Tr&Orth)
-
Ali-Asgar Najefi, FRCS, Yaser Ghani, FRCS, and Andy Goldberg, MD, FRCS
-
Ali Najefi & Karan Malhotra & Oliver Chan & Nicholas Cullen & Andy Goldberg
-
Razi Zaidi, Alexander J Macgregor, Andy Goldberg
-
Razi Zaidi, Alexander MacGregor, Suzie Cro, Andy Goldberg
-
Andrew J Goldberg, Razi Zaidi, Claire Thomson, Caroline J Doré, Simon S Skene, Suzie Cro, Jeff Round, Andrew Molloy, Mark Davies, Michael Karski, Louise Kim, Paul Cooke, on behalf of the TARVA study group
-
James Thornton, BSc, Shiraz Sabah, MBBS, BSc, MRCS, Neil Segaren, MBBS, BSc, MRCS, Nicholas Cullen, MBBS, BSc, FRCS(Tr&Orth), Dishan Singh, MBChB, FRCS(Orth), and Andrew Goldberg, MBBS, MD, FRCS(Tr&Orth)
-
A SYSTEMATIC REVIEW AND META-ANALYSIS
R. Zaidi, S. Cro, K. Gurusamy, N. Siva, A. Macgregor, A. Henricson, A. Goldberg
-
Razi Zaidi, Michael Pfeil, Alexander J Macgregor, Andy Goldberg
-
Andrew J. Goldberga,c, Alex MacGregora, Jill Dawsonb, Dishan Singhc, Nick Cullenc, Robert J. Sharpd, Paul H. Cooked

News & Blogs
The latest news as it happens
Winter '14/'15 Spring '15 Summer '15 Winter '15/'16 Autumn '16 Spring '17 Summer '17 Winter '17/'18 Spring ‘18 Winter ’18 Spring ‘19
News & Blogs
The latest news as it happens
Winter '14/'15 Spring '15 Summer '15 Winter '15/'16 Autumn '16 Spring '17 Summer '17 Winter '17/'18 Spring ‘18 Winter ’18 Spring ‘19
Privacy Notice
Use of data
Privacy Notice
Use of data
As an university, UCL uses personally-identifiable information to conduct research to improve health, care and services. As a publicly-funded organisation, we have to ensure that it is in the public interest when we use personally-identifiable information from people who have agreed to take part in research. This means that when you agree to take part in a research study, we will use your data in the ways needed to conduct and analyse the research study. Your rights to access, change or move your information are limited, as we need to manage your information in specific ways in order for the research to be reliable and accurate. If you withdraw from the study, we will keep the information about you that we have already obtained. To safeguard your rights, we will use the minimum personally-identifiable information possible.
Health and care research should serve the public interest, which means that we have to demonstrate that our research serves the interests of society as a whole. We do this by following the UK Policy Framework for Health and Social Care Research.
If you wish to raise a complaint on how we have handled your personal data, you can contact the trial team who will investigate the matter. If you are not satisfied with our response or believe we are processing your personal data in a way that is not lawful you can complain to the Information Commissioner’s Office (ICO).
The TARVA team can be contacted on tarva@ucl.ac.uk




















































